Welcome
Welcome to the UCSF TBsim tool developed by Savic Labs in support of the CPTR program funded by the Bill and Melinda Gates Foundation. This tool allows simulations of the timecourse of drug concentrations, drug actions, bacterial dynamics, and host response during drug treatment of TB. All simulations are based on a quantitative systems pharmacology (QSP) model based on data from non-clinical and clinical trials and literature.
Some notes on use of this app:
- Interfaces for either basic or advanced usage are provided, which can be switched at any time from the selector in the top-right corner. The 'Advanced' mode allows any setting to be altered, e.g. any of the QSP model parameters and all drug sensitivity and pharacokinetic parameters. The 'Basic' interface allows for more simple investigations, with standard drug regimens.
- Various existing standard-of-care drug regimens are included, while the user can also create custom regimens to evaluate their predicted outcome.
- The dashboard for population simulations allows simulations for multiple patients and to calculate expected outcome. Since population simulations are computationally intensive, these analyses are submitted to a job queue. We kindly request you to login via Google to use this functionality.
Please note that this tool is a research tool for TB experts. Do not use this tool to inform individual treatment decisions.
Contact
Please contact us at tbsim@ucsf.edu for more more information about this tool.
Single patient simulation
Simulations for single patients should not be used for research purposes. They are solely meant for illustrative purposes, i.e. to gain deeper understanding of the underlying QSP model.
Please select regimen and settings for simulation from the panel on the right, and start simulation.

Population simulation
Please select regimen and settings for the population simulation from the panel on the right, and start simulation or submit to queue.

Drug library
TB drug regimens
For initial empiric treatment of TB, start patients on a 4-drug regimen: isoniazid, rifampin, pyrazinamide, and either ethambutol or streptomycin. Once the TB isolate is known to be fully susceptible, ethambutol (or streptomycin, if it is used as a fourth drug) can be discontinued.
Patients with TB who are receiving pyrazinamide should undergo baseline and periodic serum uric acid assessments, and patients with TB who are receiving long-term ethambutol therapy should undergo baseline and periodic visual acuity and red-green color perception testing. The latter can be performed with a standard test, such as the Ishihara test for color blindness.
After 2 months of therapy (for a fully susceptible isolate), pyrazinamide can be stopped. Isoniazid plus rifampin are continued as daily or intermittent therapy for 4 more months. If isolated isoniazid resistance is documented, discontinue isoniazid and continue treatment with rifampin, pyrazinamide, and ethambutol for the entire 6 months. Therapy must be extended if the patient has cavitary disease and remains culture-positive after 2 months of treatment.
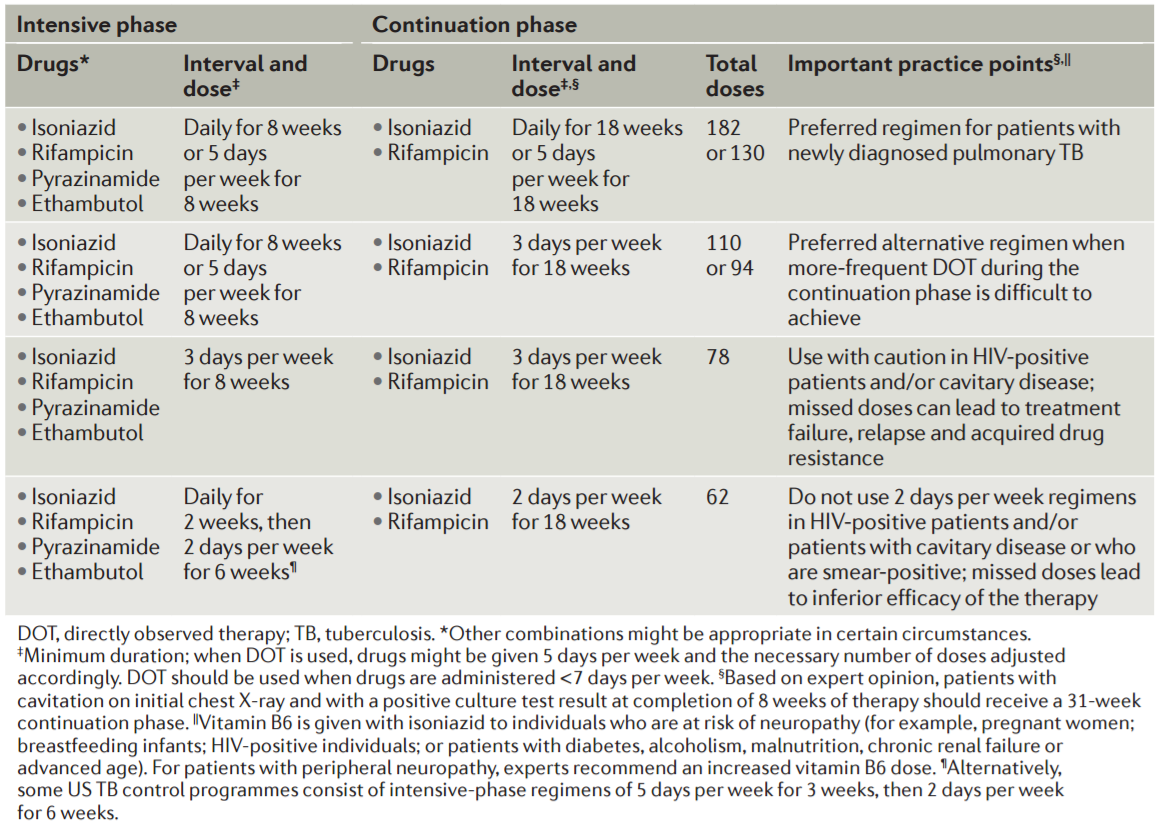
Table from: Pai M et al. Nat Rev Dis Primers, 2016.
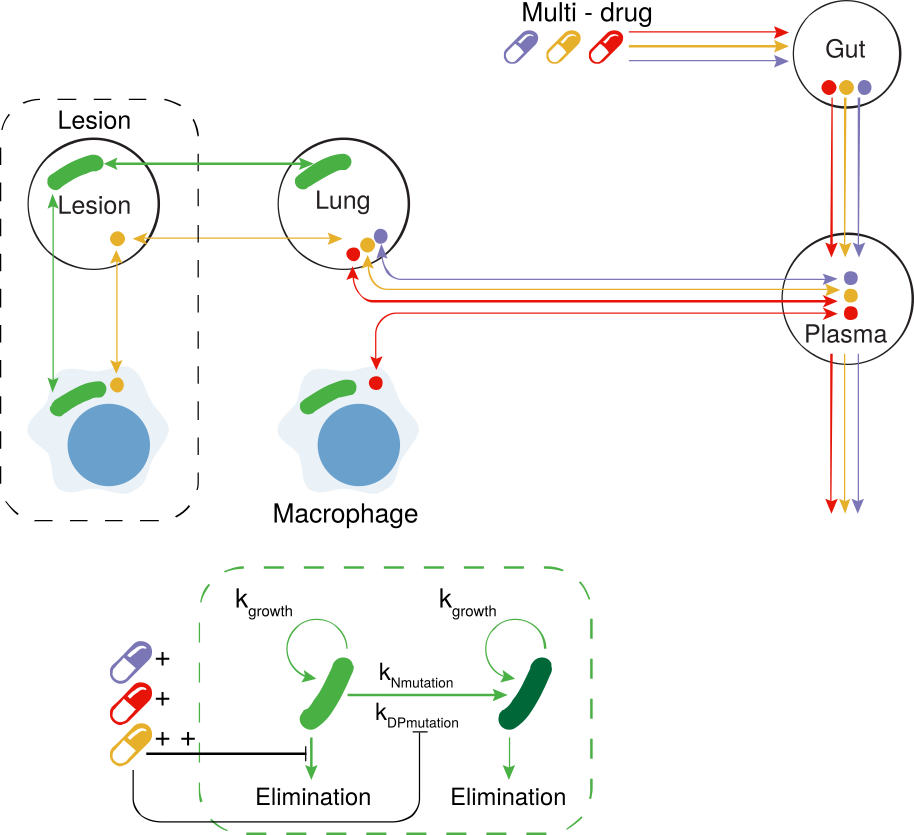
Drug regimens
Pharmacokinetics
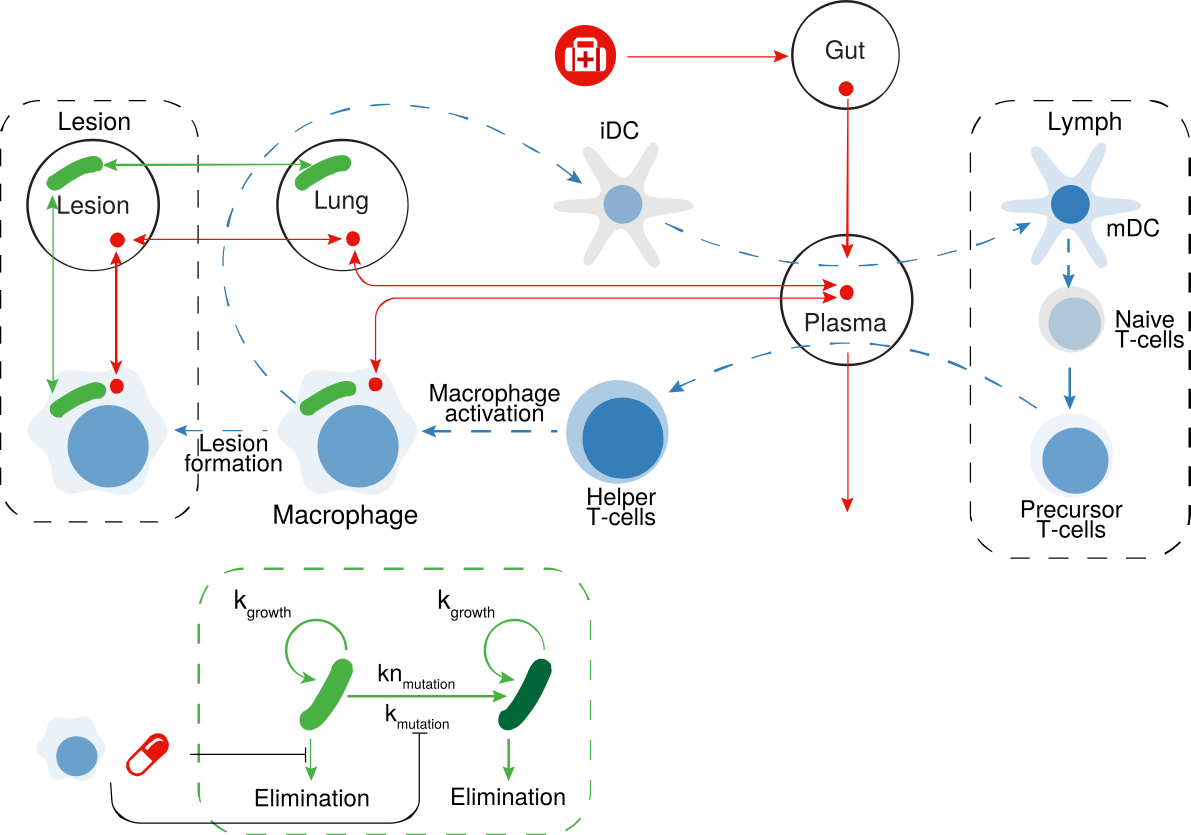
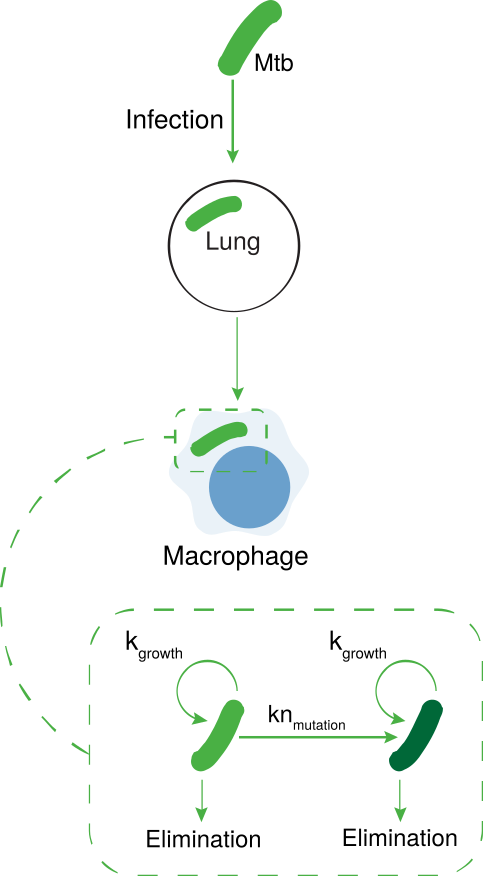
Bacterium infection
Immune response
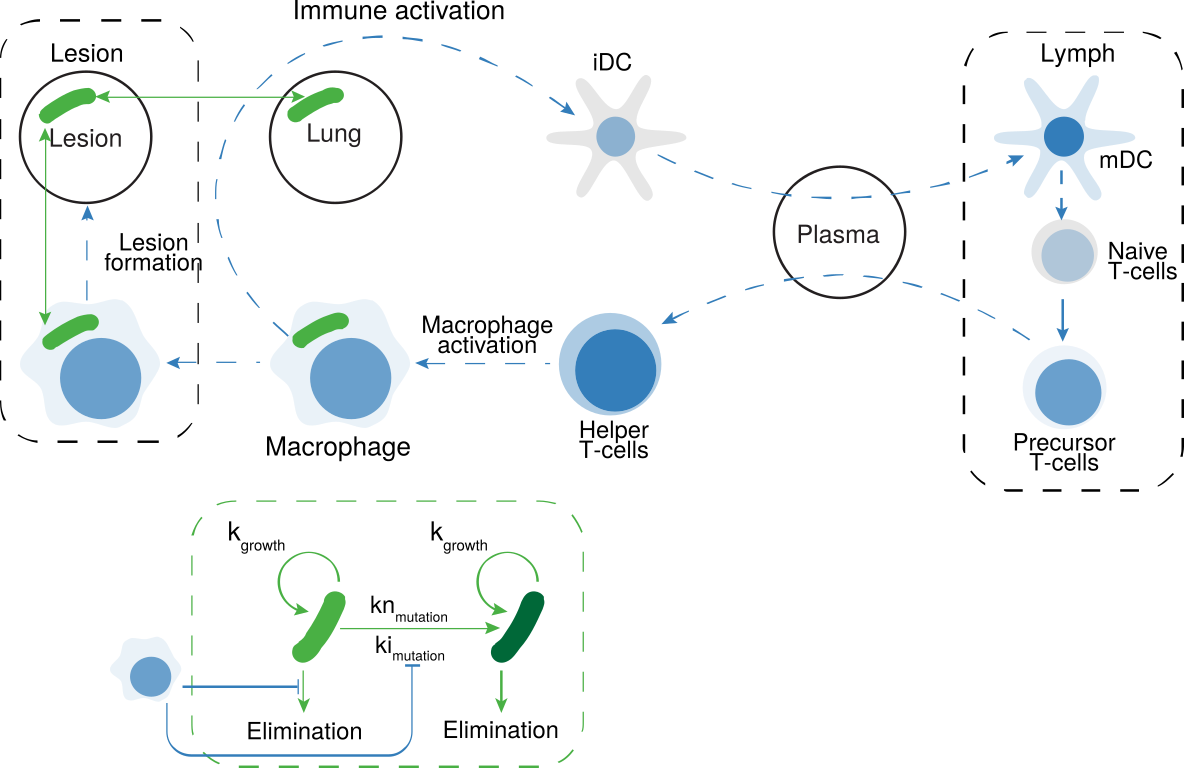
Full QSP model